The BLOG
This blog provides exclusive content for market researchers and marketing managers involved in Healthcare Marketing, prepared by APLUSA teams, and includes APLUSA important news updates.
Post ASH 23 webinar with Dr. Blin | Focus on MM session
Discover the transcription of our Post-ASH 23 webinar with Dr. BLIN, provides comprehensive coverage of several pivotal studies related to Multiple Myeloma (MM) from the ASH23 congress. Dr. BLIN delves into the specifics of each study, discussing their design, patient characteristics, main findings, and implications for the treatment of MM. Below is a condensed transcript covering the essence of each study discussed in the video!
Click to discover the MM replay video!
MULTIPLE MYELOMA
Overview
- Study Design: The PERSEUS trial is a Phase 3 study comparing the efficacy of VRd (bortezomib and dexamethasone) against Dara-VRd (daratumumab plus VD) in stem cell transplant-eligible patients with newly diagnosed MM. It explores maintenance treatment with lenalidomide alone or combined with daratumumab post-consolidation.
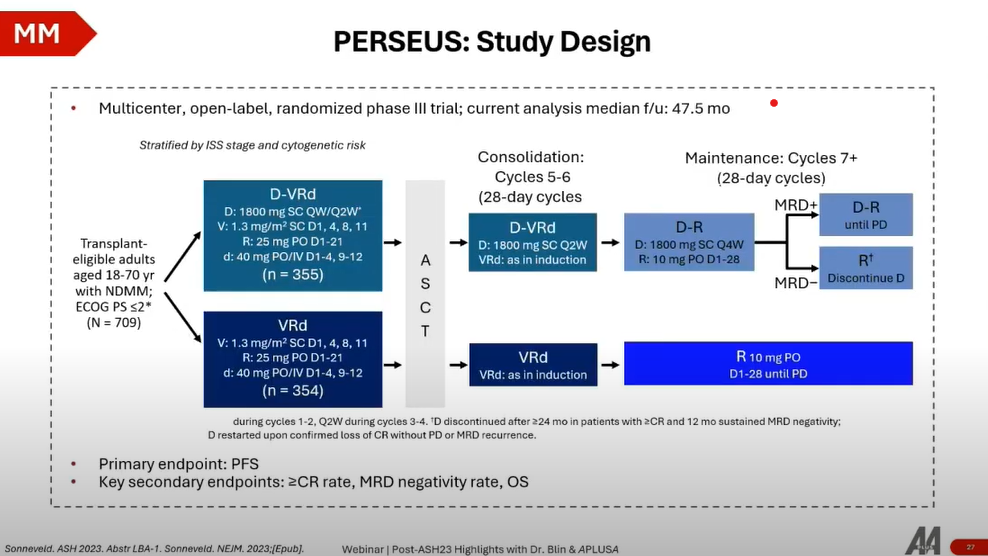
- Patient Population: The trial enrolled over 700 patients, making it a comprehensive study that spans a broad demographic of MM patients.
Key Findings
- Efficacy: The addition of daratumumab to VD significantly improved progression-free survival (PFS) at 48 months, with an 84% PFS in the Dara-VRD arm compared to 67% in the VD arm. This improvement signifies a 15% increase in the probability of PFS at four years.
- Subgroup Analysis: The benefits of Dara-VRD were observed across almost all patient subgroups, with notable improvements even in high-risk cytogenetic profiles.
- Safety: The trial reported manageable side effects, with no significant increase in toxicity observed from the addition of daratumumab to the VD regimen.
The PERSEUS trial demonstrates that the Dara-VRD induction and consolidation regimen, followed by daratumumab plus lenalidomide maintenance, could set a new standard of care for transplant-eligible MM patients. This regimen not only enhances PFS but also increases the rate of complete response and MRD negativity, potentially indicating an upcoming improvement in overall survival.
Overview
- Study Design: The European Myeloma Network (EMN) 24 trial is a Phase 3 study evaluating isatuximab in combination with carfilzomib and dexamethasone (Isa-KD) versus KD alone in stem cell transplant-eligible MM patients. It follows a treatment schema involving induction, stem cell transplantation, consolidation, and a "light consolidation" or maintenance phase over one year, with MRD negativity assessed through next-generation sequencing (NGS) as the primary endpoint.
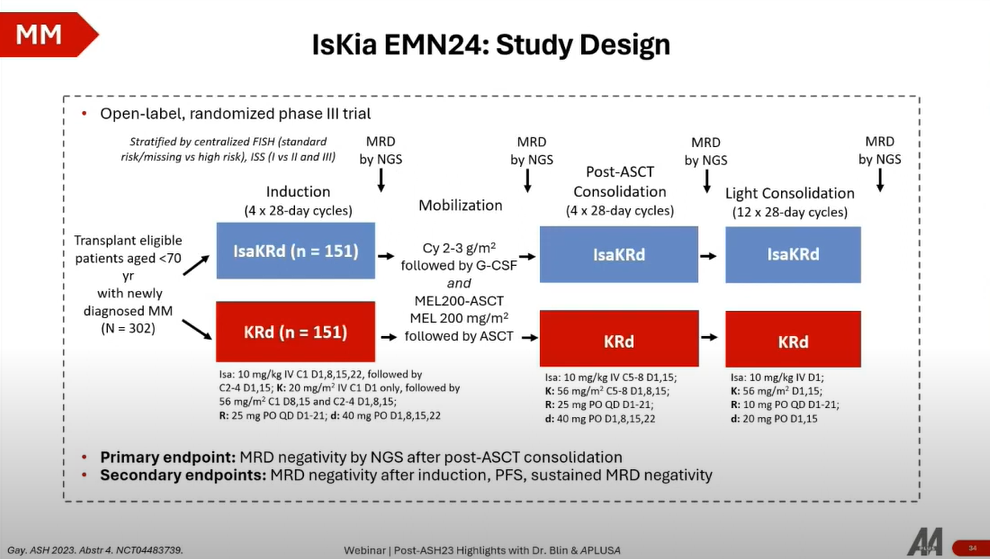
Key Findings
- MRD Negativity: The Isa-KD group showed a significant increase in MRD negativity rates, indicating a deeper response to treatment. This was evident across various thresholds of MRD assessment, highlighting the superior efficacy of the Isa-KD combination.
- Subgroup Analysis: Improvement in MRD negativity was consistent across all treatment phases and cytogenetic risk groups, affirming the benefit of isatuximab across a wide range of patient subgroups.
- Safety: The safety profile of the Isa-KD regimen was comparable to that of the standard KD therapy, with manageable side effects.
The EMN24 trial underscores the potential of isatuximab in combination with KD as a highly effective treatment for newly diagnosed, transplant-eligible MM patients. The significant increase in MRD negativity rates associated with Isa-KD suggests a meaningful impact on disease progression and possibly overall survival, advocating for the integration of isatuximab into the MM treatment paradigm.
This segment focuses on talquetamab, a bispecific T-cell engager targeting GPRC5D, evaluated in the Monumental-1 trial for its efficacy in refractory multiple myeloma patients. While showcasing high overall response and complete response rates, the study also notes significant adverse events, including skin-related issues and gastrointestinal symptoms, leading to discontinuation in a considerable fraction of patients. Dose adjustments were explored to mitigate these effects without compromising efficacy. This approach suggests that optimizing talquetamab dosing could enhance tolerability, making it a promising candidate for combination therapies in ongoing and future trials.
The phase 1/2 trial of linvoseltamab, another bispecific antibody targeting BCMA, demonstrates promising efficacy and a manageable safety profile in refractory multiple myeloma. The study reports encouraging progression-free survival rates with common adverse events like anemia, neutropenia, and cytokine release syndrome, but no significant neurologic adverse events. These findings support the advancement to the phase 3 Linker MM3 trial, assessing linvoseltamab's efficacy in a more extensive patient population, potentially offering a new treatment avenue for heavily pretreated multiple myeloma patients.




