The BLOG
This blog provides exclusive content for market researchers and marketing managers involved in Healthcare Marketing, prepared by APLUSA teams, and includes APLUSA important news updates.
Post ASH 23 webinar with Dr. Blin | Focus on MDS session
Discover the transcription of our Post-ASH 23 webinar with Dr. BLIN with Dr. BLIN, provides comprehensive coverage of several pivotal studies related to Myelodysplastic Syndromes (MDS) from the ASH23 congress. Dr. BLIN delves into the specifics of each study, discussing their design, patient characteristics, main findings, and implications for the treatment of MDS. Below is a condensed transcript covering the essence of each study discussed in the video!
Click to discover the MDS replay video!
MYELODYSPLASTIC SYNDROMES
Dr. BLIN discusses two pivotal abstracts from the ASH23 congress related to the treatment of Myelodysplastic Syndromes (MDS), focusing on advancements and clinical trial results that could potentially change the treatment landscape for patients with MDS.
- Study Design: The COMMANDS trial is a Phase 3 study comparing luspatercept (LP) with epoetin alpha (EPO) in erythropoiesis-stimulating agent (ESA)-naive, transfusion-dependent lower-risk MDS patients.
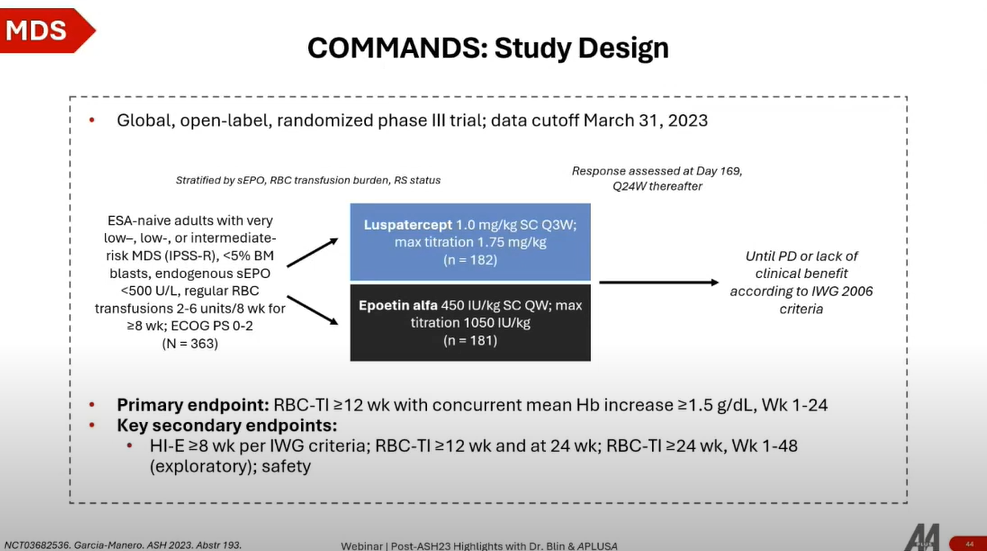
- Patient Population: Enrolled 360 patients with very low, low, or intermediate-risk MDS as per the revised IPSS, focusing on those with endogenous serum erythropoietin (EPO) levels lower than 500 units per liter.
Key Findings
- Efficacy: Luspatercept significantly outperformed epoetin alpha in achieving red blood cell (RBC) transfusion independence for more than 12 weeks, along with a concurrent mean hemoglobin increase of more than 1.5 g/dL at week 24. The benefit was observed across various patient subsets, especially notable in patients with ring sideroblasts (RS), where luspatercept showed a marked advantage.
- Secondary Endpoints: Key secondary endpoints, including hematologic improvement and reduction in transfusion needs, favored luspatercept over EPO across different timelines. The duration of transfusion independence reached in the luspatercept group was particularly impressive, suggesting potential improvements in patient quality of life.
The COMMANDS trial's results highlight luspatercept's effectiveness in managing transfusion-dependent lower-risk MDS patients, especially those with ring sideroblasts. Its superiority in achieving longer periods of transfusion independence and potential quality of life improvements positions luspatercept as a significant advancement in MDS treatment.
The Imerge Phase 3 trial represents a significant study focusing on the efficacy of imetelstat, a telomerase inhibitor, in treating patients with Myelodysplastic Syndromes (MDS) who are either refractory to or ineligible for erythropoiesis-stimulating agents (ESAs) due to high serum erythropoietin levels (>500 mU/mL).
Overview
- Study Design: This trial enrolled 178 patients in a 2:1 randomization to compare imetelstat versus placebo. The stratification was based on transfusion burden and IPSS-R category. Imetelstat was administered intravenously every four weeks, with patients also receiving supportive care as needed.
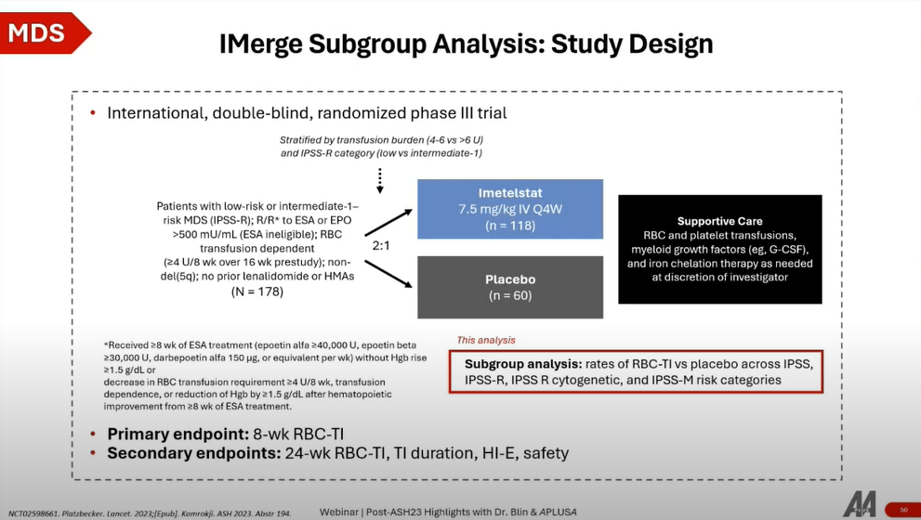
- Patient Population: Targeted at MDS patients in a second-line setting or later, particularly those unresponsive or relapsing after ESA treatment, or first-line patients with serum erythropoietin levels indicating ESA ineligibility.
Key Findings
- Efficacy: Imetelstat demonstrated a clear advantage in achieving durable transfusion independence compared to placebo, across various timelines (weeks 8, 16, 24), highlighting its potential as an effective treatment option.
- IPSS-R Risk Categories: The response to imetelstat was significantly better across low to intermediate IPSS-R risk categories, except in the low-risk group at more extended timelines, where the difference was not statistically significant.
- Molecular Risk Categories: Using the next-generation IPSS-M, which incorporates high-risk mutations like TP53, imetelstat showed superiority over placebo in improving outcomes across all molecular risk subgroups, affirming its broad applicability.
The Imerge trial positions imetelstat as a potentially new standard of care for lower-risk, transfusion-dependent MDS patients who have limited options due to ESA refractoriness or ineligibility. The results indicate that imetelstat could offer a meaningful clinical benefit, emphasizing the need for further research, possibly phase 3 trials, to fully establish its role in MDS treatment paradigms. This trial underscores the evolving landscape of MDS treatment, focusing on targeted therapies that address specific mechanisms of the disease.
If you missed our previous sessions on Multiple Myeloma, Diffuse Large B-Cell Lymphoma, and Acute Myeloid Leukemia, you can now read our blog posts by clicking the buttons below:





