The BLOG
This blog provides exclusive content for market researchers and marketing managers involved in Healthcare Marketing, prepared by APLUSA teams, and includes APLUSA important news updates.
Post ASH 23 webinar with Dr. Blin | Focus on AML session
Discover the transcription of our Post-ASH 23 webinar with Dr. BLIN, providing comprehensive coverage of several pivotal studies related to Acute Myeloid Leukemia (AML) from the ASH23 congress. Dr. BLIN delves into the specifics of each study, discussing their design, patient characteristics, main findings, and implications for the treatment of AML. Below is a condensed transcript covering the essence of each study discussed in the video! Click to discover the AML replay video!
Click to discover the AML replay video!
ACUTE MYELOID LEUKEMIA
Dr. BLIN provides an in-depth analysis of a key abstract from the ASH23 congress, focusing on Acute Myeloid Leukemia (AML). Here's a brief overview of the points covered in the first chunk of the transcript:
- Trial Design: The Quantum First study is a phase 3 randomized clinical trial focusing on first-line AML patients with FLT3 ITD mutations, comparing standard chemotherapy (3+7 regimen) plus placebo versus quizartinib (a FLT3 inhibitor) in combination with the standard chemotherapy.
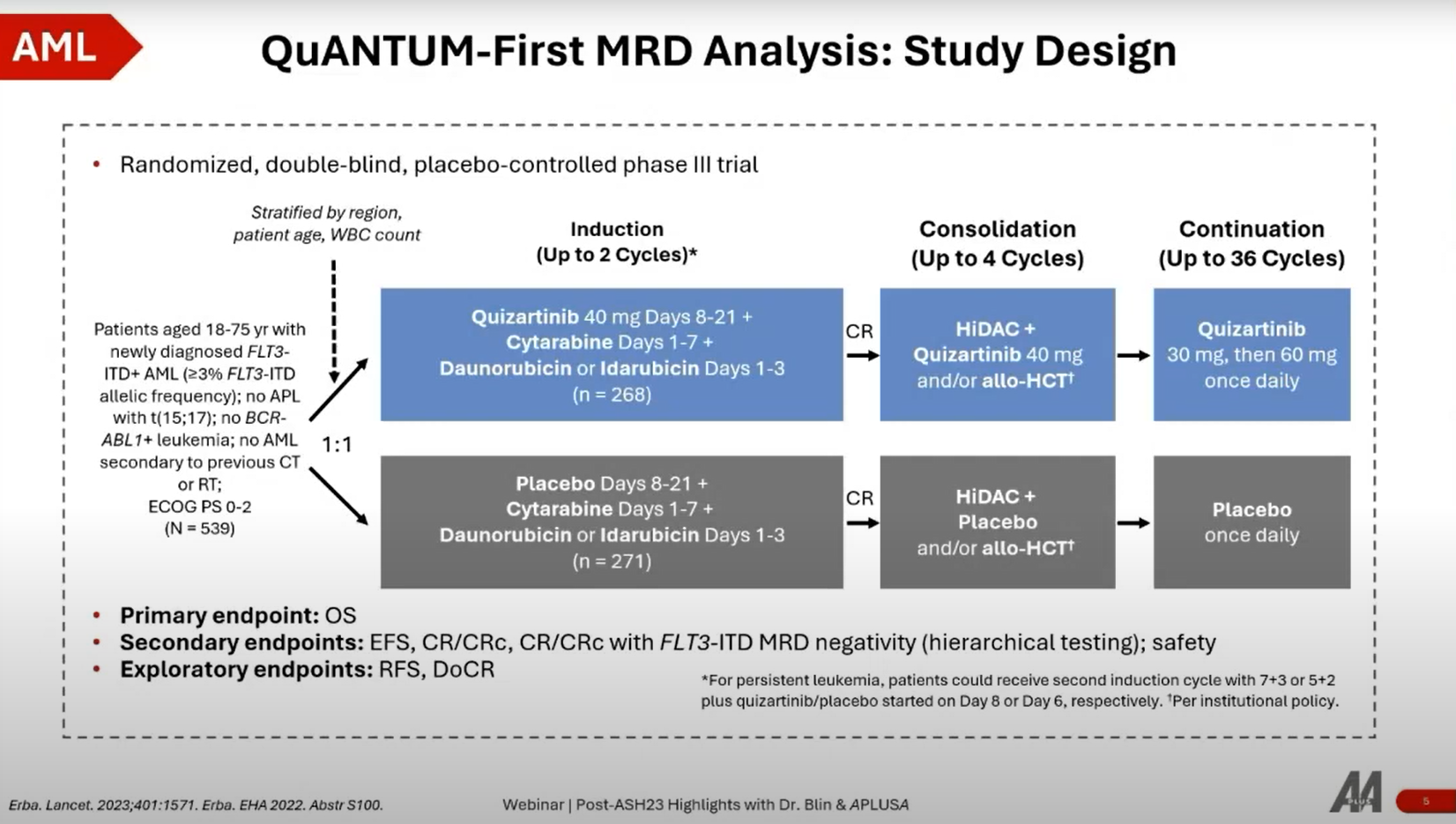
- Objective: The primary endpoint is overall survival, with secondary endpoints including FLT3 ITD mutation clearance (MRD negativity) assessed through PCR testing.
- Patient Characteristics: All participants were eligible for intensive chemotherapy and had FLT3 ITD mutations at various levels. The study ensured a balanced comparison between the quizartinib and placebo groups.
Key Findings
- MRD Negativity and Survival Correlation: The study found a strong correlation between FLT3 ITD MRD clearance and improved overall survival. The hazard ratios were significant across different MRD thresholds and timelines within the treatment process.
- Impact of Quizartinib: Treatment with quizartinib led to deeper responses and more frequent FLT3 ITD MRD negativity compared to placebo, indicating a clear association between undetectable FLT3 ITD MRD and longer overall survival.
Dr. BLIN highlights the prognostic value of FLT3 ITD MRD clearance under quizartinib in patients eligible for intensive chemotherapy, showcasing how achieving undetectable FLT3 ITD MRD is associated with significantly improved survival outcomes. This abstract underscores the importance of targeted therapy in enhancing treatment efficacy for AML patients with specific genetic mutations.
2. Phase I/II Trial of Quizaritinib, Venetoclax, and Decitabine in FLT3-ITD-Mutated AML
Overview
- Trial Design: A phase 1/2 trial focused on patients with FLT3 ITD mutated AML who are ineligible for intensive chemotherapy. It investigates the combination of quizaritinib (a FLT3 inhibitor) with venetoclax and azacitidine.
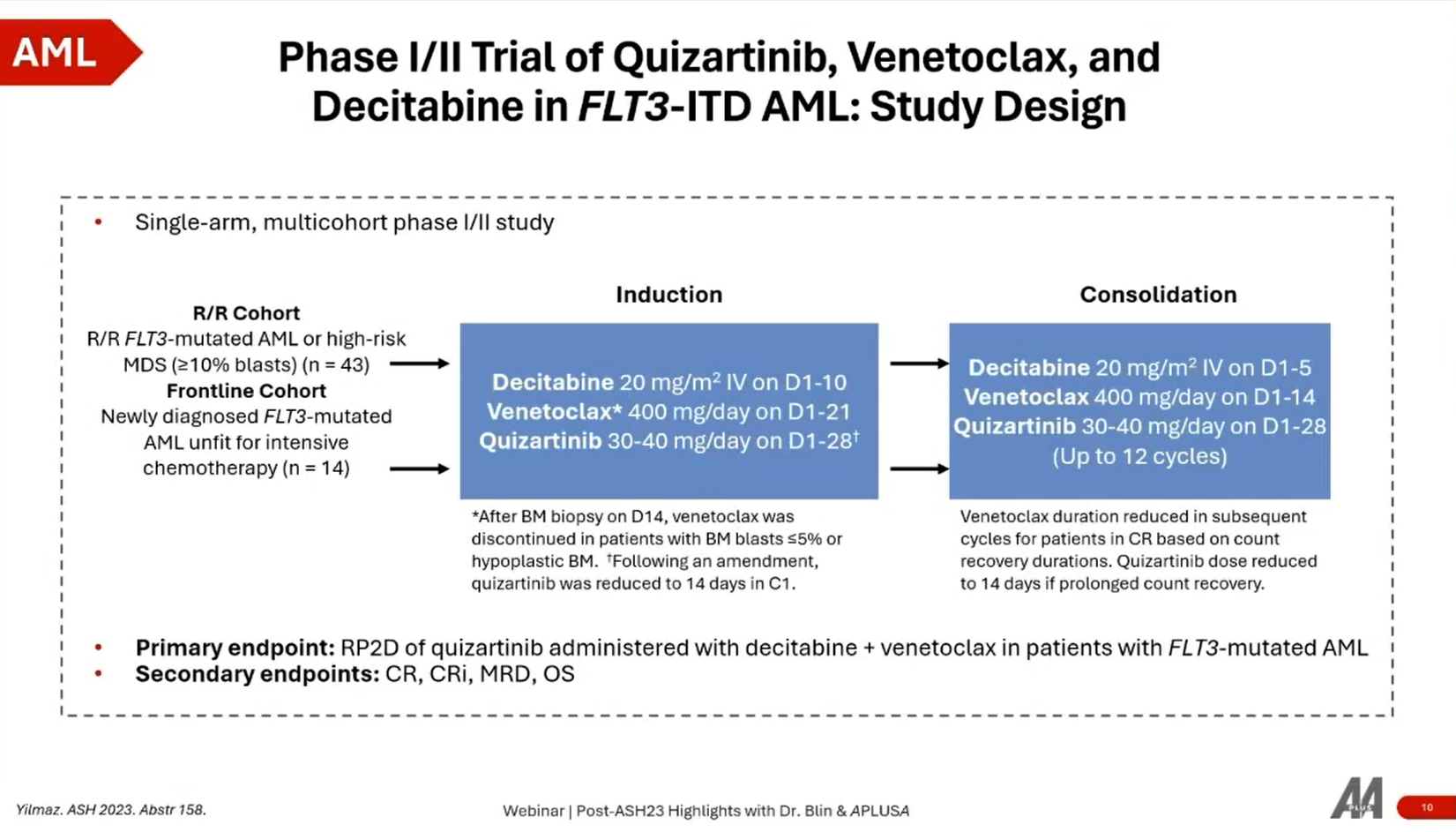
- Patient Cohorts: The study includes two cohorts: relapsed/refractory and frontline, with a total enrollment of 57 patients.
- Objective: The primary endpoint is to determine the recommended phase 2 dose of quizaritinib, with secondary endpoints including CR (Complete Remission), CRi (Complete Remission with incomplete hematological recovery), and overall survival.
Key Findings
- Efficacy: The CR/CRi rate is 65% in the intent-to-treat population, indicating similar responses regardless of previous exposure to gilteritinib (another FLT3 inhibitor).
- Safety: Side effects include delayed neutrophil recovery, which can be mitigated by dose adjustments, suggesting that the combination is manageable and promising.
The study demonstrates the efficacy of quizaritinib in combination with venetoclax and azacitidine for AML patients’ ineligible for intensive chemotherapy. Achieving a CR/CRi rate of 65% indicates a promising treatment option, with manageable side effects through dose adjustments. This combination represents a significant advancement for a challenging patient demographic, highlighting the potential for future phase 3 trials.
3. AUGMENT-101: Phase II Study of Revumenib for R/R KMT2Ar Acute Leukemia
Overview
- Trial Design: Phase 2 study of revumenib (a MENIN inhibitor) targeting patients with relapsed or refractory AML or ALL, particularly those with KMT2A rearrangements or NPM1 mutations.
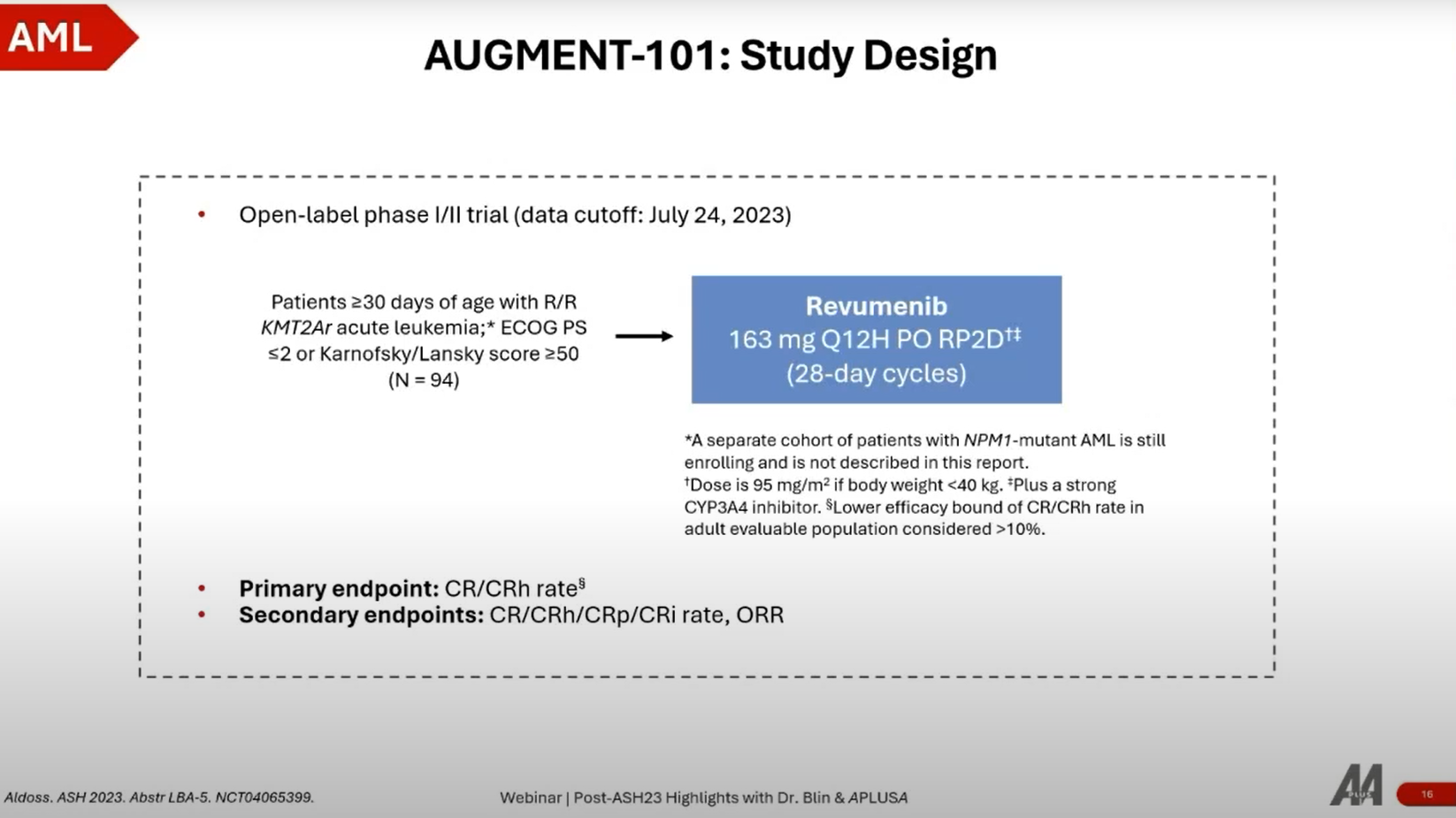
- Patient Population: Enrolled nearly 100 patients, focusing on those with difficult-to-treat mutations and a history of multiple treatment lines.
Key Findings
- Response Rates: A 63% overall response rate with revumenib as monotherapy, and a 23% CR/CRi rate, showcasing significant efficacy in a heavily pretreated patient population.
- Overall Survival: Median overall survival was eight months, with patients achieving CR or CRi rapidly, usually within less than two months.
Subgroup Analysis
- Primary Refractory Patients: Notably relevant results in this challenging patient group, indicating the potential of revumenib in treating patients with primary refractory disease.
- KMT2A Rearrangements: Responses were observed across various KMT2A rearrangement subgroups, suggesting the broad applicability of revumenib for patients with this genetic marker.
The Augment 101 trial showcases revumenib's potential as a monotherapy in treating relapsed or refractory AML or ALL patients with KMT2A rearrangements or NPM1 mutations. With a 63% overall response rate and rapid achievement of CR/CRi, revumenib offers a new, effective treatment pathway for heavily pretreated patients, underscoring the importance of targeted genetic therapies in leukemia treatment strategies.
Study Design
- The research is based on a retrospective analysis from the French FOW Center, exploring outcomes related to the discontinuation of venetoclax (Ven) in AML patients who achieved CR or CRi.
- It addresses a critical question in AML management regarding the potential benefits and risks associated with halting venetoclax treatment, a key component in the treatment regimen for many AML patients.
Key Findings
- Survival Outcomes: The main conclusion from the STOP-VEN study is that overall survival (OS) in patients who discontinued venetoclax after achieving remission was not negatively impacted compared to those who continued treatment. This finding suggests that for some patients, particularly those in remission, discontinuing venetoclax does not compromise survival outcomes.
- Side Effect Management: Discontinuing venetoclax may also offer benefits in terms of reducing the burden of treatment-related side effects, such as hematologic toxicities and gastrointestinal issues like diarrhea and fatigue. This could potentially improve the quality of life for patients in remission.
The STOP-VEN study provides valuable insights into the management of AML remission, particularly regarding the use of venetoclax. By demonstrating that stopping venetoclax in certain remission patients does not adversely affect survival outcomes, the study supports a more personalized approach to AML treatment. It highlights the importance of weighing the benefits of continued treatment against the potential for side effects, suggesting that for some patients, a strategy of discontinuing venetoclax could be viable.
5. STIMULUS-AML2: Preliminary Safety of Sabatolimab Monotherapy in Patients With MRD+ AML after AlloSCT
Study Design
- Objective: To evaluate the safety and efficacy of sabatolimab in AML patients who have undergone ASCT and are either experiencing relapse or have positive MRD, a high-risk factor for relapse.
- Treatment Approach: Sabatolimab targets the TIM-3 pathway, aiming to reactivate the patient's immune system to target and eliminate leukemic cells effectively.
Key Findings
- Safety Profile: One of the critical observations from the STIMULUS-AML2 study is the optimal tolerance reported for sabatolimab, with minimal severe adverse effects. Notably, the treatment did not induce graft-versus-host disease (GVHD) or other immune-related adverse events, which are significant concerns in post-transplant therapies.
- Efficacy Indicators: Preliminary efficacy data suggest that sabatolimab may delay relapse in patients with MRD positivity after ASCT, indicating its potential to improve long-term outcomes in a subset of AML patients at high risk of relapse.
The STIMULUS-AML2 trial presents sabatolimab as a promising immunotherapeutic agent for AML management post-ASCT, especially for patients with residual disease. The lack of GVHD and immune-related adverse events with sabatolimab is particularly encouraging, as these are common challenges in post-transplant care.
The potential of sabatolimab to delay relapse and improve survival in AML patients post-ASCT could represent a significant advancement in AML treatment, offering hope for better management strategies in the high-risk post-transplant setting. Further studies and dose expansion cohorts are underway to validate these findings and potentially incorporate sabatolimab into the AML treatment paradigm, especially for patients with MRD positivity after ASCT.
This study underscores the importance of novel immunotherapeutic approaches in AML, focusing on enhancing the immune system's ability to combat relapse and improve patient outcomes in the challenging post-transplant phase.
To conclude, Dr. Blin discussed five key abstracts from the ASH23 congress, focusing on Acute Myeloid Leukemia (AML). The Phase III QuANTUM-First Trial demonstrated the efficacy of quizartinib in improving overall survival and achieving FLT3 ITD MRD negativity. Additionally, the Phase I/II Trial of Quizartinib, Venetoclax, and Decitabine showed promising responses in FLT3 ITD mutated AML patients ineligible for intensive chemotherapy. The AUGMENT-101 Study highlighted the effectiveness of revumenib monotherapy for heavily pretreated patients with specific genetic mutations. The retrospective STOP-VEN Study indicated that discontinuing venetoclax in certain remission patients does not compromise survival outcomes and may reduce treatment-related side effects. Lastly, the STIMULUS-AML2 Trial showcased the potential of sabatolimab to delay relapse and improve outcomes in high-risk post-transplant AML patients with MRD positivity. Dr. Blin emphasized the significance of these findings, underscoring the importance of targeted therapy and novel immunotherapeutic approaches in advancing AML treatment and improving patient outcomes.
If you missed our previous sessions on Multiple Myeloma, Diffuse Large B-Cell Lymphoma, and Myelodysplastic Syndrome, you can now read our blog posts by clicking the buttons below:









